Health Canada is advising Canadians that two lots of a brand of Acetaminophen are being recalled due to a labelling error that may lead to overdose and, in severe cases, death.
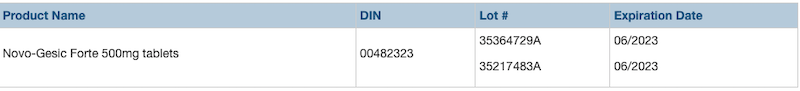
Teva Canada is recalling two lots of Novo-Gesic Forte/Acetaminophen tablets, sold in 500 mg tablets, due to a labelling error that could result in a person exceeding the maximum daily dosage for acetaminophen, explains a news release.
The label on the bottle of affected products incorrectly states, “do not take more than 4,000 mg (12 tablets) in 24 hours.” However, the number of tablets should be eight based on the maximum daily dose of 4,000 mg, not twelve.
If consumers follow the incorrect directions, they could ingest doses of acetaminophen ranging from 4,500 to 6,000 mg (nine -12 tablets) in 24 hours and experience symptoms of acetaminophen overdose.
Signs of acetaminophen overdose include nausea, vomiting, lethargy, sweating, loss of appetite and pain in the upper part of the abdomen or stomach. Abdominal pain may be the first sign of liver damage and may not be apparent for 24 to 48 hours. Liver damage may result in liver failure or, in the most severe cases, death.
The affected products were distributed in Canada starting Aug. 3 and Health Canada is monitoring the company’s recall.
What you should do
- Stop using the recalled products and return them to the pharmacy/store where they were purchased.
- If you think you or a family member has taken too much acetaminophen, call your local right away.
- Consult a health care professional if you have used any of these products and have questions.
- Contact Teva Canada by calling 1-800-268-4129, or emailing [email protected], if you have questions about the recall.
- Report any health product-related or to Health Canada.